Yates Account
Join now
Create a Yates account today!
Sign up to join the Yates Garden Club for monthly e-mails packed with seasonal inspiration, tips for success & exclusive promotions.
Plus if you’re a Garden Club member you can take part in the Yates Growing Community - a blog to share successes, get advice & win prizes in fun challenges along the way!

Forgot password
Enter the email address associated with your account, and we'll email you a new password.

Do you have plants that look a bit sad? Are they yellowish or sickly, or not responding, no matter how much you feed and care for them? The answer might lie beneath them, in the soil.
Soil is the crucial foundation for plant growth, so if there’s something not quite right with it, plants can suffer. A key factor in understanding soil is pH – the measure of alkalinity or acidity of the soil. pH significantly impacts on soil quality and plant wellbeing.
What is soil…and what makes it healthy?
Soil is so much more than just dirt. It’s a living ecosystem, made up of microorganisms and larger soil organisms, minerals, organic matter, air and water. The natural minerals that make up the soil heavily influence the levels of organisms, organic matter, air and water in that soil.
The different particles that make up soil include clay, silt and sand, which all result from thousands of years of grinding and weathering of solid rock. Clay, silt and sand are graded by particle size, with clay being the smallest and sand the largest. They all have different physical characteristics and behaviours.
It’s the varying percentages of these particles in soil that make up soil texture. For example, a heavy clay soil has very little sand and silt in it; due to the structure and minute size of the clay particles there’s very little room for air, water and soil organisms in the tiny pores between the clay particles. Without these key ingredients, clay soil is considered poor for growing. On the other hand, when the mix of these elements are well-balanced it makes for a highly fertile soil that’s perfect for planting. Even if your soil is considered poor – high in clay, or high in sand – it can always be improved. Soil pH also plays a part in soil health; if it isn’t in the optimum or ideal range, plants won’t grow well.
What is soil pH?
pH is short for ‘potential of hydrogen’. It’s a measure of the concentration of hydrogen in a water-based substance (in our case, the substance is soil). The more hydrogen ions soil contains, the more acidic it is. As the hydrogen content decreases, the soil becomes less acidic.
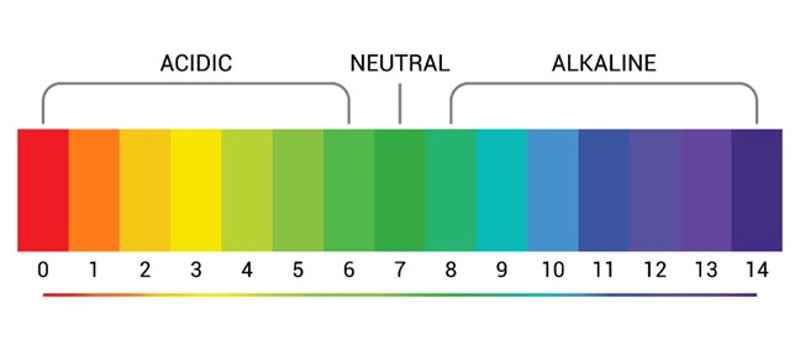
pH is measured on a scale from 1 to 14, with 7 being neutral. Anything above 7 is considered alkaline. Anything below 7 considered acidic. Plain water (H2O) sits right in the middle of the scale, with a neutral pH of 7.
Usually, soil pH sits somewhere between 3.5 and 10. A measurement of between 6.5 and 7.5 is considered neutral for soil. Most plants thrive in that neutral pH band, but they can suffer when it’s too high or low. But there are also eccentric plants that do things their own way, actively preferring to grow in ‘sweet’ (alkaline) or ‘sour’ (acidic) soil. Making sure plants are growing in their favoured pH range is really important for long term plant health and productivity.

What determines soil pH?
Soil pH can be quite varied. It mostly depends on what parent material the soil was formed from (e.g., limestone, granite, basalt). Different climates weather these parent materials at different rates, with temperature and rainfall having a big influence (e.g. warm environments with high rainfall, like NZ, have a higher rate of acidification from intensified leaching). Soil pH also varies from changes it’s been subjected to over time, e.g. farming, fertiliser practices, loss of vegetation or changing topography.
Almost all soils in New Zealand are acidic, except for Central Otago, the region surrounding Lake Ellesmere, Hawkes Bay, Gisborne and the Far North, where the soil is neutral or slightly alkaline.
Soil pH in New Zealand is strongly influenced by vegetation cover. The most acidic soil conditions are found in National Parks or conservation areas with untouched native bush.
In the South Island, the entire West Coast has acidic soil, especially in areas North of Hokitika, reaching up as far as Kahurangi National Park (where the pH can be as low as 4.0).
In the North Island, areas in and around the Pureora Forest, Te Urewera and the Tararua ranges are strongly acidic, usually measuring below 5.0. The Kaimanawa, Kaweka and Ruahine Forest Parks, along with the Wainuiomata region are even more acidic, measuring as low as 4.0.
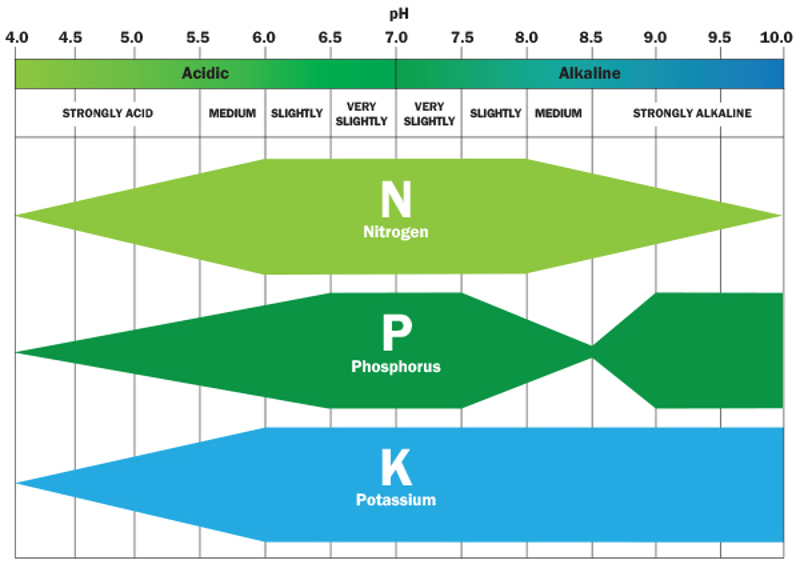
pH effect on nutrient availability in soil
What does soil pH mean for my plants?
pH has a big effect on a plant’s ability to absorb vital nutrients from the soil. If pH is too acidic or alkaline, this can stunt or restrict root growth. This naturally limits the plants water and nutrient uptake. In a worst-case scenario where the pH is extreme, it makes major nutrients (nitrogen, phosphorus and potassium) unavailable to plants, along with locking up many essential micronutrients. The chart above illustrates the effect of pH on nutrient availability. Without adequate nourishment, plants naturally decline and may ultimately die off.
Adding fertilisers and trace elements to soil can help, but these are only temporary solutions. It’s best to measure the soil pH and tackle any issues at the source.
How to test soil pH
The easiest way to test soil pH is by using a soil pH test kit, which you can purchase at your local nursery or garden centre. You can also use a soil pH meter, but we think the best value for home gardeners is a cheaper kit with litmus paper test strips. It’s best to take a few different samples from the area to get a good average of your soil pH. Test and compare a sample from the surface with a sample from a depth of about 7cm, then take the average as your pH measurement for that spot.
Ideally, it should sit between 6.5-7.5, but if it’s on either side of this range, then you’ll need to improve it with specific soil conditioners, like liquid sulfur or lime.
It’s important to note that adjusting soil pH doesn’t happen overnight, it’s more of an ongoing process to reach the desired pH. Continual cultivation of soils and addition of synthetic fertilisers can make soil more acidic, so it’s a good idea to check your soil pH regularly and make adjustments as needed.
How to make soil acidic, or lower soil pH
If your soil is sitting above 7.5, ideally, you’d lower or acidify it. The most effective way to lower soil pH is to add Yates Soil Acidifier Liquid Sulfur, which contains elemental sulfur (S). Soil bacteria convert the sulfur to sulfuric acid, which helps lower the soil pH. This process is highly dependent on soil bacteria, so it’s best to apply it when conditions are warm. For best results, apply sulfur every 4 weeks until the desired pH is achieved. Take care not to overapply – putting more on won’t make the process any faster; overdoing it can end up causing issues with sulfur toxicity, or cause the pH to drop too low. Changing soil pH isn’t a fast process, it requires patience while the soil microbes do their thing.
Adding aluminium sulfate is a faster way to decrease soil pH. We have Yates Liquid Aluminium Sulfate, which is ideal for blueing hydrangeas or acidifying soil in a localised area around a plant. However, it does need to be applied carefully: overdoing it can lead to issues with aluminium toxicity. A build-up of aluminium in the soil has a detrimental effect on plant health and vigour, so if you’re looking for a long-term solution, we’d recommend Yates Soil Acidifier Liquid Sulfur for the job. Always remember when acidifying soil: gently does it, little by little gets you there.
Using vinegar to lower soil pH isn’t recommended. It causes immediate and localised sharp drops in pH, but the adjustment doesn’t last because the acetic and phenolic acids in vinegar are organic compounds; they biodegrade very quickly and the soil returns to its former pH.
How to make soil more alkaline, or increase soil pH
If your soil pH is sitting below 6.5, it’s a good idea to increase it (make it more alkaline). Adding Yates Thrive Naturals Garden Lime (containing calcium carbonate) or Yates Thrive Naturals Dolomite Lime (contains calcium carbonate and magnesium carbonate) is the most effective way to reduce soil acidity. When calcium or magnesium carbonate is applied to acidic soil, the ions separate into calcium/magnesium, and carbonate. The calcium and magnesium (both valuable micronutrients) bind to the soil, which makes them available to be taken up by the plant when required. The carbonate binds with the excess hydrogen in the soil to form water and carbon dioxide. Using up hydrogen in the conversion directly reduces the acidity of the soil.
The easiest way to sweeten soil is with Yates Hydrangea Pinking Liquid Lime & Dolomite, which is a mix of calcium carbonate and magnesium carbonate in a convenient liquid form. Apply every 3-4 weeks until the desired pH is reached.
Acid loving plants
These plants like acidic soil and grow better when the pH is lower. Ideally, soil pH should be between 5-6.5. Any lower and this can inhibit nutrients and stunt plant growth.
- Azaleas
- Rhododendrons
- Gardenias
- Camellias
- Blueberries
- Hydrangeas
Alkaline tolerant plants
These plants are more tolerant of sweeter or alkaline soils, so will still thrive if the pH is slightly on the higher side. A soil pH range of 7.5-8.5 is best. Any higher and this will impact plant health.
- Buddleja
- Lavender
- White correa (Correa alba)
- Viburnum tinus
- Pride of Madeira (Echium candicans)
- Geranium
- Rosemary
- Hibiscus
- Bauhinia
- Hydrangea
















Share
Share this article on social media